How to Find Empirical Formula
32 16 16 64. For example while the molecular formula for glucose is C 6 H 12 O 6 its empirical formula is CH 2 O showing that there are twice as many hydrogen atoms as carbon or oxygen atoms but not the actual numbers of atoms in a single molecule or how they are.

Determining Empirical And Molecular Formulas Chemistry Tutorial Youtube Chemistry Education Chemistry Molecular
Calculate the empirical formula of the compound.

. Multiply all the subscripts in the empirical formula by the whole number found in step 2. Multiply all the subscripts in the empirical formula by the whole number found in step 2. Suppose you have a compound of aluminum oxide if the mass of the aluminum is 4151g and the mass of the oxygen is 3692 g.
Multiply each of the moles by the smallest whole number that will convert each into a whole number. Divide by the lowest number of moles. In aluminum oxide there is 1 mol of aluminum and 2 moles of oxygen.
Join millions of learners from around the world already learning on Udemy. I show you how to work out the empirical formula from the molecular for. This video goes into detailed steps on how to find the empirical formula of a compound.
Add up the atomic masses of the atoms in the empirical formula. Divide the molar mass of the compound by the empirical formula mass. 304g N 1 1 mol N 1401g N 217 mol N 217 1 mol N.
7 Use the scaling factor computed just above to determine the molecular formula. From the empirical formula you can work out the molecular formula if you know the relative formula mass M r of the compound. The result should be a whole number or very close to a whole number.
The steps are1 Write the atoms involved in the calculation2 Write the mas. The result is the molecular formula. Combine the moles of each atom into an empirical formula.
This chemistry video tutorial explains how to find the empirical formula given the mass in grams or from the percent composition of each element in a compoun. To find the moles. Ad Find the right instructor for you.
Hooray for no more confusionCheck out my NEW complete guide on Empir. Since the atomic mass of aluminum is 2698 and for oxygen is 1600. Divide the molar mass of the compound by the empirical formula mass.
For Acetylene the empirical formula is C 2 H 2. Molecular formulas and empirical formulas. The result should be a whole number or very close to a whole number.
How do you find the actual and empirical formula. The non-whole number empirical formula of the compound is Fe 1 O 15. Best PDF Fillable Form Builder.
Now multiply each value with the smallest integer that can convert 25 into a. Any compounds chemical formula can be defined using one of two types of formulas. The simplest type of formula called the empirical formula shows just the ratio of different atoms.
Find the number of moles of each element in the compound by dividing the mass in grams of each with its atomic mass. Even if youre only asked to find the molecular formula. How do you find the actual and empirical formula.
For Acetylene the empirical formula is CH. Also our online empirical formula calculator considers these equations for finding the simplest positive integer ratio of atoms present in a compound chemistry. 6 Divide the molecule weight by the EFW 6407 64 1.
FeO 2 115 23. Find the simplest formula. So the simplest formula of the compound is CH2O.
An empirical formula represents the simplest whole-number ratio of various atoms present in a compound. The molecular formula shows the exact number of different types of atoms present in a molecule of a compound. Divide each value by the lowest figure.
Both the empirical formula and the molecular formula represent the atoms number and identity. The result is the molecular formula. In this video I explain to you what is meant by the empirical formula of a compound.
SO 2 times 1 gives SO 2 for the molecular formula. In this article we will study in detail the empirical formula and how to calculate it. Ad Edit Fill eSign PDF Documents Online.
Assume 100g so we have 304g N and 696g O. Since the moles of O is still not a whole number both moles can be multiplied by 2 while rounding to a whole number. A compound is found to contain 6480 carbon 1362 hydrogen and 2158 oxygen by weight.
Choose from many topics skill levels and languages.

Calculating Empirical Formula Chemistry Math Formula

Trick To Find Out Empirical Formula Molecular Formula Class 9 10 11 12 Youtube How To Find Out Molecular Chemistry

Empirical And Molecular Formula Notes Chemistry Worksheets Teaching Chemistry Chemistry Notes

The Empirical Formula Of A Compound Is The Simplest Formula That Gives The Correct Relative Numbers Of Teaching Chemistry Chemistry Lessons Chemistry Classroom

Determining Empirical And Molecular Formulas Chemistry Tutorial Youtube Chemistry Education Chemistry Molecular
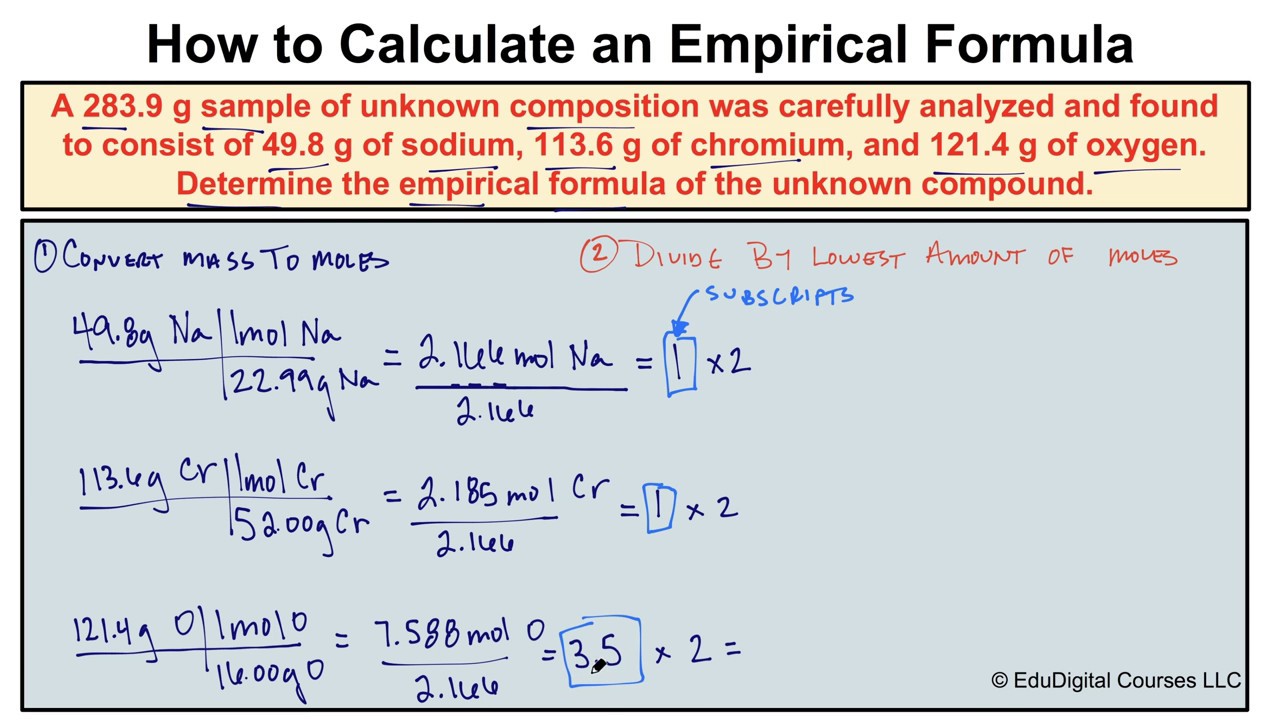
How To Calculate An Empirical Formula Chemistry Worksheets Chemistry Notes Scientific Method Worksheet
Comments
Post a Comment